Quite the title for the post, no? We just had a paper accepted in Nature Scientific Reports entitled Predictive computational modeling to define effective treatment strategies for bone metastatic prostate cancer and we thought we would share the news with you. This paper is part of our ongoing work with the Lynch lab at Moffitt to better understand the evolutionary dynamics of prostate cancer metastases in the bone. Continuing on our previous integrated computational platform [blog, description, paper] we decided to investigate a novel treatment which is not typically used in the clinic: the inhibition of the key signaling molecule TGF-Beta. If you know anything about prostate cancer research you will find TGF-Beta familiar. It has been studied to death and not much of clinical use has ever been found. The issue is that although we know a lot about it, cancer biologists have not found a model to integrate all those findings. We decided to use a computational agent-based model to integrate a lot of what we know of the biology of TGF-Beta in bone metastatic prostate cancer.
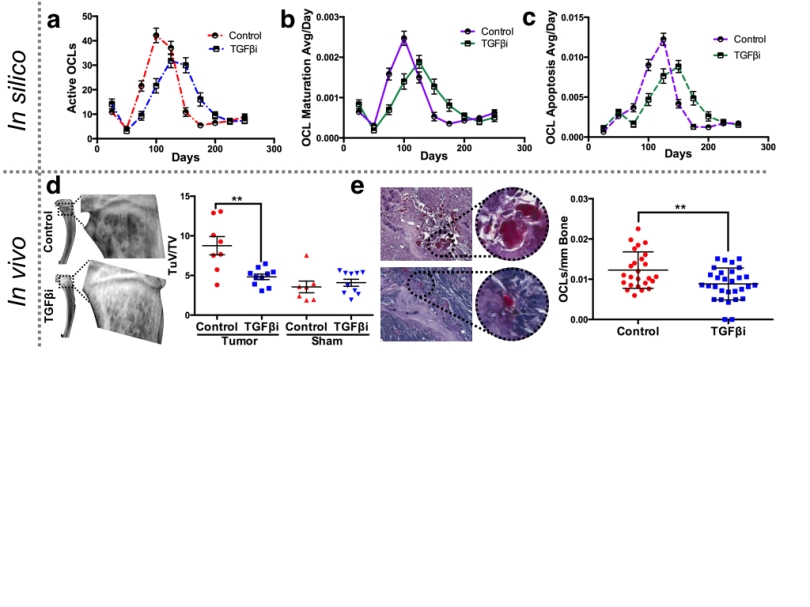
We took our previous model and worked carefully to make sure all the cell types responded to TGF-Beta to the best of the cancer biology community’s knowledge. We then went back and forth between this computational model and a mouse model in order to make sure that we could recapitulate TGF-Beta inhibition in the context of both: normal bone and cancerous bone. As you can see in the figure, one advantage of the computational model is that resolution of information about each cell type in any point of the tumor at any stage of the progression. This information can be contrasted with the relatively scarce experimental one to asses whether there are disagreements that need to be resolved.
Another advantage of the computational model is that, even if it is relatively complex like this one, it can test hypothesis at a much faster rate, for longer periods of time (and in a much cheaper and humane fashion) than mouse models. This allowed us to explore all kinds of schemes for the application of the TGF-Beta inhibitor. Once something promising is found we can then test those schemes experimentally to see how they fare with the mouse model.
What we found is that pre-application of the inhibitor is a much better option than application once the metastasis has established itself in the bone. What this could mean to a potential patient using a TGF-Beta inhibitor (which as said, is not a clinical option at the moment) is that we could give these treatments after the primary tumor has been identified in the prostate but before metastases have been found. It is important to understand though that this is the result of basic research and that this conclusion will have to remain hypothetical while we find how move our approach from a pre-clinical model to a clinical one. More importantly, what we have shown is that the integration of various sources of experimental data into a purposely made mechanistic mathematical model with the right amount of complexity (not too simple, not too complex) allows us to identify and explore novel treatments in ways (and at speeds) that would not be possible otherwise.